Note: this article has two primary purposes. First, it is a compilation of all the existing safety and toxicology data on DMSO for anyone planning to utilize it in a clinical setting. Second, it is meant to serve as a place to collect reader’s (often incredible) experiences with DMSO so individuals who are considering using DMSO can have testimonials to juxtapose with the wealth of data I am gradually presenting on DMSO.My time in the medical field has led me to accept many medical practices are adopted because of politics or economics rather than because existing evidence shows they work. Nonetheless, certain instances of this happening still astound me to this day, particularly the blacklisting of DMSO (dimethyl sulfoxide) as:•This simple chemical is incredibly safe and effective and treats a wide range of challenging medical conditions that impact millions that still lack an effective therapy (outside of DMSO).
•Because of its efficacy, once discovered, it took the country by storm, resulting in millions using it, the scientific community getting behind it and publishing thousands of studies on DMSO, numerous pharmaceutical companies making large investments to bring it market, professional athletes promoting it, numerous governors, congressional representatives and senators (on behalf of both themselves and their constituents) pressuring the FDA to give it a fair chance for decades and state legislatures independently legalizing it because the federal government would not.
•Many approved pharmaceutical products take advantage of DMSO’s properties to work (e.g., in those products, DMSO is often classified as an inert “vehicle”). Similarly, DMSO is FDA approved for one condition (interstitial cystitis) and is approved for a wide variety of veterinary uses (e.g., the same conditions it treats in humans).
•Over the past 40 years, more than 10,000 articles on the biological implications and 30,000 articles on the chemistry of DMSO have appeared in the scientific literature—much of which, as I’ve shown here is remarkably compelling and paradigm shifting in healthcare.
•Yet, despite all of that, DMSO was effectively erased from history. It is now widely seen as an unproven and dangerous therapy, and even within the natural health field, most people do not know it exists.
Because of all that, I’ve felt a responsibility to use this platform to get the knowledge on DMSO out, which I began by presenting the strong case that DMSO is an incredible therapy for:•Circulatory disorders like Reynaud’s and varicose veins.•A wide range of neurological disorders, including ischemic and hemorrhagic strokes, and spinal cord injuries leading to paralysis or dementia.•Allowing patients who’ve had decades of chronic pain (from a variety of different causes) to get their lives back.•Healing a wide range of injuries (e.g., sports injuries, traumatic impacts) and chronic musculoskeletal problems (e.g., spine and shoulder issues) and wounds (e.g., burns or surgical incisions).•Chronic rheumatic conditions (e.g., arthritis).•Complex protein disorders (e.g., amyloidosis).•Down Syndrome.
In turn, I’ve received numerous reports from readers (I’ve been gradually sharing here) from readers who’ve experienced rapid life-changing benefits from DMSO, very similar to the data I provided, which showed DMSO had an 80-90% success rate in treating.
Yet, despite all of this, I’ve still only touched the tip of the iceberg of what can be done with DMSO (e.g., in upcoming articles I will also review how DMSO is also quite helpful for a variety of eye, ear, dental, gastrointestinal, and autoimmune conditions such as tinnitus and macular degeneration, along with also having remarkable utility in treating cancer, challenging infections and debilitating collagen disorders). As a result, I’ve also received numerous queries from readers who inadvertently discovered many of those benefits when they used DMSO for a chronic pain condition.
For example, some of the more recent reports I’ve received include:
After AMDs articles, I used DMSO on an acute bruise and it completely took the pain away AND resolved the swelling that was developing. 🤯🤯🤯🤯. It’s hardly even tender today. Incredible
Dear MWD, you are so right on learning to doctor yourself. I don’t travel without DMSO, ivermectin and aspirin. Two nights ago at bedtime I developed chest pains that radiated between my shoulder blades. Being in New Mexico (Oh, Lord, don’t let me die in New Mexico) I put DMSO along my carotids on my neck and took 2 aspirin. In an hour the pain was gone and I slept soundly. Scared the hell out of my poor husband.
After reading this I got a tub of 70/30 gel and applied it to my sons feet three times per day. He was riding his skateboard barefoot and crunched his toes under his feet. No broken bones.
Within three days he said he felt no pain or discomfort at all. For the sort of injury it was it seemed like the sort of thing which would take weeks to stop hurting and for all discomfort to end for a sixteen year old!
Excellent research - I've given DMSO to my mom and it has helped her arthritis immensely.
I am an 81 year old woman who was injured by the first of a series of 2 Shingrix shots in 2019. I never took the second shot. Eight days after receiving that shot I developed excruciating pain in my arms, hands, legs and feet. Although being told by two doctors that the vaccine did not cause the pain, the neurology team at a major medical institution diagnosed my condition as acute inflammatory demyelinating polyneuropathy(AIDP), on the spectrum of Guillame Barre. They treated me with gabapentin which relieved the pain. However, I was left with neuropathy in my feet which caused severe and painful spasms in my feet along with numbness on the bottom of my feet. After several weeks seeing a neurologist, I asked her what could be done to help this situation. She said there was nothing. After this article I started using DMSO on the bottom of my feet and over the tops of my toes when I went to bed. The first time I used it, I had no spasms which always happened at night when I was trying to go to sleep. I’ve now used it for 3 days and still no spasms. It’s like a miracle. I’ll continue using it to see if it helps resolve the numbness in my feet. God bless you, AMD. I never would have tried this without your articles and would have suffered needlessly forever. I owe you a great debt. Thank you. I’m telling everyone about DMSO and sending your articles as well. Your contributions are, without doubt, some of the most important I have read.
Likewise, a grateful reader reported their wife (a retired nurse) had a fall that injured her back and left her in severe pain and unable to walk which chiropractic did not help, and then a few days later, the ER could not help either. However, rather than accept an admission to the hospital, she took DMSO, her back worked itself out, and she was spared months of recovery with the standard of care.
Note: I’ve also received reports on a variety of other conditions (e.g., one subscriber shared a DMSO mixture shrunk their hemorrhoid), and another shared DMSO has gradually been shrinking their cataract.
If we take a step back, it should be clear that DMSO should be in widespread use, but instead something very wrong happened with DMSO which resulted in it getting blacklisted. This was due to the FDA continually doubling-down on an unshakable ideological crusade against DMSO that I believe ultimately resulted from the FDA not wanting to lose its grip over the practice of medicine in the United States (as the therapeutic potential of DMSO greatly threatened the FDA’s ability to control how medicine was practiced).
In turn, I believe what happened is a critical story to be told because:
•The entire story of DMSO is a remarkable example of thousands of dedicated scientists and doctors giving everything they could to bring this critical innovation to the public and thus highlight the incredible potential our scientific apparatus has to address the problems that plague humanity. In contrast, because of the decades of rigid suppression of independent science, we’ve become habituated to science being unable to solve our basic problems—something that urgently needs to change.•The FDA’s gross misconduct with DMSO set the stage for what the agency continues to do to this day, and helps to explain why so many remarkable treatments have been withheld from the public while dangerous and ineffective ones are continually pushed upon the public (e.g., consider what happened throughout COVID-19).
Is DMSO Safe?
Throughout the FDA’s war against DMSO, the FDA has always cited two reasons to justify its conduct.•That no evidence existed DMSO worked, which as I showed in the first and second part of this series, was an absurd claim as data from thousands upon thousands of patients showed DMSO worked dramatically better than the existing therapeutic options.•That DMSO was an incredibly dangerous drug that it was critically important to protect the public from—something I’ve argued was a patent lie.
Note: these lies now extend far beyond America. For example, this posting by Health Canada, beyond characterizing DMSO as a dangerous solvent, makes numerous demonstrably false claims about DMSO and declares no evidence exists for DMSO’s efficacy—which is extraordinary given how many of clinical trials have proven DMSO works and how easy many of those studies are to locate.
Furthermore, beyond the above points being absurd, the existing standards within the FDA are that if unmet medical needs exist or there is no viable cure for a serious illness, those standards can be loosened (hence why the COVID vaccines were approved, or more recently, why an incredibly unsafe and ineffective Alzheimer’s drug was approved despite the FDA’s outside panel vetoing it and resigning in protest once the FDA overrode them. In the case of DMSO, this is particularly relevant as many of the diseases DMSO was proven to treat (e.g., Down Syndrome, Spinal Cord Injuries, Scleroderma) are severe illnesses that have remained incurable for decades.
All of this thus raises the question. How safe is DMSO? Since that data is relevant to both understanding the FDA’s crusade against it and likewise for anyone considering using it, I have done my best to compile all of that data here..
The Safety of DMSO
No drug is completely safe. However, I consider DMSO to be one of the safest drugs I know of for a few key reasons.1. It was subject to intensive scrutiny and a wide range of toxicology studies (as the FDA was desperate to find a reason to justify their prohibition on it). Nonetheless, nothing was found.
2. Rather than be toxic to cells, cells can tolerate very high concentrations of DMSO and in many cases, DMSO can protect cells from dying or rescue ones that were in the process of dying. All of this is extraordinarily unusual.
3. A large number of animal studies (in at least 11 different species—including fish) have shown a lack of toxicity for DMSO.
4. Clinical trials consistently show a lack of toxicity from DMSO.
5. DMSO does not accumulate in the body, so it has no cumulative toxicity.
6. Millions of people have used DMSO, many of whom have used it for years if not decades (e.g., taking it daily for over 50 years). Still, despite this (outside of a few easily preventable mishaps which will be discussed below), no serious issues have emerged.
For context, DMSO has a safety profile that is orders of magnitude greater than drugs that are routinely taken without a thought being given to their safety.
I will now attempt to summarize all the pertinent data I’ve found on DMSO’s safety. Some of this may sound concerning, but it needs to be seen in the context that it was found by using very high doses of it, as an immense amount of research was devoted to finding any possible way DMSO could be toxic (something rarely done for most drugs) and as a result, much of this is not applicable to how most of you will use DMSO.
Note: while this is a bit lengthy, I felt it was important to share all the toxicology data I could locate so that I did not inadvertently filter any potentially useful information and create a biased or misleading reference.
Median Lethal Dose (LD50)
One of the most commonly used methods to determine a substance’s toxicity is to see how high a dose of it needs to be given to kill 50% of the exposed animals (which leads to countless tragic and, in my eyes unnecessary animal deaths each year). Part of why this value is needed is because each drug has both a toxic dose and an effective dose, and the goal is to find something in between those two that can be prescribed to people

In turn, when the therapeutic index is narrower, the drug is harder to use without side effects and often is given in more controlled settings (e.g., at an IV infusion center) so it is less likely someone will accidentally get a toxic dose. Conversely, drugs with a wide therapeutic index require less oversight in their administration.
Note: one of the major problems with how medicine is practiced now is that (in order to make drugs easily marketable products) standardized doses are always used. This in turn results in many patients receiving inappropriate doses (e.g., ones that are too high), and both I and my colleagues thus believe one of the most critically important forgotten arts of medicine is knowing how to chose an appropriate dose (a subject which I discussed in further detail here).
Since there was so much controversy around DMSO, an immense amount of LD50 data was obtained that showed DMSO is far less toxic than a variety of commonly used substances.
Note: as toxic doses approaching the DMSO’s LD50 were used in animals, tissue injury would also occur (e.g., vein irritation, vasoconstriction and necrosis after intravenous application, hemorrhagic gelatinous and edematous lesion at the site of muscular or subcutaneous injections, or liver damage)—much of which was thought to be due to osmotic injuries to the tissues created by the high concentrations of DMSO. However, if the animals survived, this damage typically went away within a week.
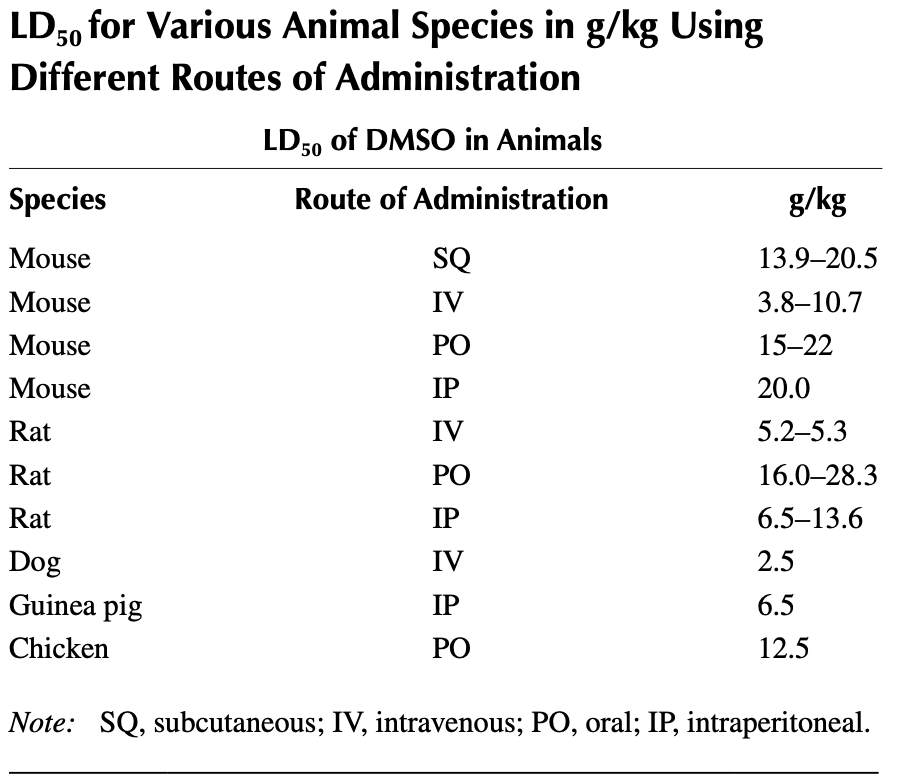
In short, to reach the LD50 of DMSO, you would need to drink roughly two quarts of it within an hour, which is more DMSO than daily DMSO users ingest over two months.
For comparison, many commonly used substances are 10-100 times as toxic as DMSO:

Note: LD50s are typically written in mg/kg rather than g/kg due to their higher toxicity. Additionally, some variation exists in the LD50s for the substances listed above (hence why I attempted to present an approximate range).
Additionally, none of the previously cited LD50 studies assessed topical applications of DMSO. This is because a limit gets reached as to how much DMSO can be absorbed through the skin, and that amount is far below the LD50 (e.g., in a previous article I cited cases of people who were going to lose a limb or finger which was then soaked in DMSO and the only side effect they experienced was the tissue fully recovering).
In mice, the LD50 of topical DMSO was estimated to be 50g/kg, as mice survived being dipped (immersed) up to their necks in up to 60% DMSO, while rats survived being dipped in up to 80% DMSO, or up to 60% DMSO three times per week for 26 weeks—with the dipping sessions often lasting 24 hours.Note: the main changes observed in the repeatedly dipped rats were ulcerous dots on the belly and back, eye changes (lens clouding and near-sightedness) and slight changes in the blood and liver—all of which were reversible. Conversely, when 100% DMSO was painted over their entire body each day, no adverse effects occurred (which again demonstrates that the toxic dose was quite high).
In humans, it is not practical (or ethical) to dip them in vats of DMSO all day long, but the closest approximation of that was attempted (subjects were repeatedly fully covered with DMSO gel so they could receive 1g/kg a day of DMSO—a dose 3-30 times higher than what is typically used by patients) and then monitored for 90 days. Despite this extraordinarily high dose being received each day, no toxicity was observed (whereas with virtually any other drug on the market, serious issues would emerge from repeatedly receiving that high of a dose)
Note: in monkeys, the LD50 of topical DMSO was established to be over 11g/kg, while the oral LD50 was established to be over 4g/kg.
In addition to the LD50 studies, a variety of other safety studies were also done on animals which likewise found (through an extensive battery of tests) that DMSO had negligible toxicity. For both length considerations, and because I don’t believe many of you want to hear about all the other grotesque animal studies that were done to appease the FDA, I am not listing and summarizing them here. However, for those researchers who are interested, the two best resources I’ve found on DMSO’s toxicology are this textbook on the pharmacology of DMSO (which has a lengthy discussion about the existing toxicology data and can be read on the internet archive here) and this well-referenced 2019 book that was written by two of the leading pioneers of DMSO.
Since I have read through approximately 100 studies that stated a similar side effect profile (DMSO was safe and typically caused the same reactions at comparable rates), rather than list each of them, I will just share the most pertinent information.
Note: one of the most detailed summaries of DMSO’s animal toxicology data can be found here.
Common Side Effects
Two side effects are frequently seen with DMSO usage that often decrease with successive applications of DMSO:•A temporary (and sometimes uncomfortable) irritation of the skin when DMSO is administered topically that typically disappears in 10 minutes (and at most 20) and varies widely in how it feels (e.g., some find it pleasant, others find it extremely unpleasant). Typically this irritation can be alleviated by immediately washing it off with water, and it is generally advised to avoid further irritating the skin by scratching the irritated area.
Studies find this irritation affects 50-85% of users (particularly blonde or red haired and fair skinned individuals or those already prone to skin reactions), and is more common at higher concentrations or when gels (rather than liquid DMSO) are used. Because of this, it is typically advised to not use more than 70% DMSO topically (outside of emergencies like a stroke) although many (e.g., readers here) tolerate 100% DMSO without issue. In roughly 15% of patients this reaction is “marked,” in 3.5% it is enough that the patients stop using DMSO (with those reactions clearing within 10 days of stopping DMSO and the clearing being accelerated with topical hydrocortisone), and in 0.1% of patients the reaction is severe enough that it requires suspending the treatment. Additionally, in some patients, repeated applications to the same area can cause drying and scaling of that skin (which will heal in time but also responds to aloe vera). Finally, when DMSO is ingested orally, a much lower concentration needs to be used to avoid irritating the GI tract.Note: while some people are fine with the taste of oral DMSO, most find prefer to mix it with something else to mask its flavor.•When DMSO is metabolized, if the body is unable to fully oxidize it (e.g., due to reductive stress) some of it instead is reduced to dimethyl sulfide, which in turn is excreted through the skin and lungs (and hence the breath), leading to a significant number of DMSO users (but not all of them) developing a characteristic garlic or clam like odor that typically lasts for a few hours but in some cases can last for up to 72 hours. Because of this side effect, DMSO users who experience it typically structure their social life and when they take DMSO so that the odor will not occur at inconvenient times (e.g., when they wish to have physical intimacy with their spouse).Note: this odor increases with greater doses of DMSO.
In turn, with the exception of one headache, every negative response to DMSO a reader here has reported here was either this odor or skin irritation.
Severe Side Effects
The most significant danger of DMSO is having an allergic reaction to it (e.g., generalized body rashes). Compared to most drugs, this effect is fairly rare (estimates range from 1 in 1000 patients to 1 in 2000 patients), and fortunately has not been documented to lead to severe allergic reactions that can be fatal (e.g., in a sample of 2000 patients, 2 patients experienced minor difficulties with breathing that quickly reversed with treatment). Nonetheless, it is generally advised to check for an allergic reaction to DMSO (the process for which is described here) before beginning significant topical use of DMSO or internal use of it.Note: DMSO has not been shown to create allergic tendencies (e.g., it didn’t create sensitivities to pollens in the environment)—which for instance is one of the major issues with certain childhood vaccines.
The other significant effect of DMSO is that prior to it drying, it will drag chemicals (but not bacteria) which are on the skin where it is applied to the body. Incidents of this nature are extremely rare, and typically, it occurs when someone was in the vicinity of a pesticide (which was on their skin and resulted in them getting ill), but I have also heard of a few more severe cases like this one:
My Dad told us of an adverse event related to DMSO use during his working career: lab technicians liberally used DMSO’s excellent solvent characteristics to clean glassware. One technician was a heavy smoker and immersed a hand in DMSO. Almost immediately he had a severe reaction and was rushed to the hospital where he almost died. He was found to have severe nicotine poisoning … the DMSO transmitted the nicotine stains from his fingers directly into his bloodstream.
Note: I have read a few reports of individuals who typically didn’t react to DMSO having significant reactions to DMSO when it was applied to parts of the body (e.g., the hair) where other compounds were present. For this reason, it is generally a good idea to always clean an area before applying DMSO to it, wait until DMSO dries (which takes about 20 minutes) before letting anything (e.g., synthetic clothing) contact the skin and to use clean (e.g., purified) water when diluting DMSO.
That all said, deaths from DMSO are incredibly rare, and despite millions of people using it, only three deaths have ever been associated with it.
The first (in 1965) involved an Irish woman who had been on a course of antibiotics and an anti-anxiety medication who continued to use DMSO despite having an allergic reaction to it, and then died of what was reported to be an anaphylactic reaction. It was never determined if DMSO was the responsible agent for her death.
The second case came from oral DMSO and was reported at this conference (but I could not find any additional information on this “overdose” beyond what was listed in FAERS report 13555640).
The final case is a still unsolved medical mystery where a woman with end-stage cervical cancer (who was also taking DMSO), presented the the ER, died shortly after (from cervical cancer) but simultaneously sickened many of the workers around her (e.g., 3 fainted around her, 5 required hospitalization, with 1 being in the ICU for 2 weeks). One theory put forward was that the medical oxygen and electrical shocks she received caused the DMSO in her to be converted to dimethyl sulfate, a theory many chemists then disagreed with (hence making it an unsolved mystery). I personally believe this theory is impossible as she was tachycardic at the start (whereas a DMSO overdose slows the heart rate) and because the metabolite of DMSO that is exhaled (dimethyl sulfide) and hence what would have been in contact with the medical oxygen, unlike DMSO, cannot react to become dimethyl sulfate. Rather, if DMSO was at fault, I believe it is much more likely a contaminant was present in the DMSO she got from the hardware store.
In comparison, far more deaths can be conclusively linked to almost every other pharmaceutical on the market.
Moderate Side Effects
DMSO often reduces the toxicity of another pharmaceutical (e.g., it makes chemotherapy less damaging to the rest of the body), but in some cases it can instead enhance the toxicity or strength of the pharmaceutical. At the time when this was researched, it was shown to occur with alcohol and barbiturates due to altering their metabolism and DMSO’s parasympathetic enhancing effects, but it likely occurs with other drugs as well (e.g., benzodiazepines). However, to the best of my knowledge, no other potentiating effects have been observed.
Additionally, a study evaluating the effect of DMSO on the Shwartzman phenomenon (tissue necrosis which occurs following the repeated introduction of a toxin to the body) injected a bacterial toxin (LPS) under the skin and then followed it with an IV injection of LPS. If DMSO was applied topically after the first injection, the reaction at the injection sites was enhanced following the second LPS injection, while if DMSO was applied topically after the second injection (when the severe Shwartzman phenomenon would occur) DMSO prevented the reaction, but if IV DMSO was given after the first injection, no change occurred, but when IV DMSO was given after IV LPS, all 6 rabbits died within 2 hours.This is one of the only examples I have come across of DMSO making something become significantly more dangerous (with the other being that if carbon tetrachloride was fed to rats with a feeding tube, injecting DMSO into the abdomen made it more toxic), but given how many drugs DMSO could interact with, it’s quite possible other interactions exist (e.g., DMSO makes both antibiotics and chemotherapy more effective and simultaneously makes chemotherapy less toxic).
In turn, I’ve received numerous questions on if a harmful interaction exists between DMSO and anticoagulants like Eloquis (leading to excess bleeding) or metal prostheses (leading to their components being leached into the body). I can see numerous reasons arguing for why DMSO might be harmful, beneficial or inconsequential in each case and to the best of my knowledge, no harmful interactions have been reported in any of these cases—but unfortunately, since neither issue has been extensively studied (except when DMSO is mixed with stem cells), I can’t actually state with confidence there isn’t an interaction.
Note: while DMSO has strong anti-platelet activity (detailed here), I have come across a few papers that mentioned that while DMSO prevented dangerous clots, it did not affect the blood coagulability of subjects. The most detailed paper I found assessing this question found DMSO had the typical U-shaped curve of a zeta potential restoring agent, which meant at very low doses it caused blood to gel together, at most doses it dispersed it, and at high doses (which would not be found during medical DMSO therapy) it clumped blood together, along with also having a U-shaped curve of the recalcification time—all of which led the authors to conclude DMSO probably has an inconsequential effect on blood clotting, except possibly when it reached low levels as it was being eliminated from the body (where in practice it has not actually been shown to cause clotting).
Simultaneously, in the places I would have expected to see the other drug reactions be listed, they weren’t. For example, this was part of a memo Merck sent out to their clinical investigators on September 8, 1965, summarizing their experiences with approximately 4,000 patients who had received DMSO anywhere from once to daily for 18 months in a list that is fairly representative of the side effects seen now:
Approximately 85 percent of patients experience a typical histamine-type reaction at the site of application, usually transient mild itching and burning and some erythema. This is not considered to be a true adverse reaction to the drug but a typical side effect. A fine vesiculation, occasionally at the site of application, is also usually transient. After prolonged administration, drying, mild wrinkling and occasionally some scaling of the skin is not uncommon. This is no worse than after a mild sunburn.
A few cases of generalized dermatitis have occurred. This is usually a wheal and erythema reaction of a histamine type occurring at sites distant from the area of application. Rarely may this generalized dermatitis be so severe. The drug should be discontinued if a generalized dermatitis develops.
Rarely, serious or potentially serious hypersensitivity reactions may occur. One fatal reaction has been reported in a patient who continued to receive the drug after signs of extreme sensitivity developed.
There has also been a report of laryngeal edema of a mild degree in one patient.
Other unusual reactions have included hypotension in a few patients.
A few cases of mild paresthesias have been noted. Re-evaluation of most of these cases has shown that these were in patients with a strong emotional overlay. Elimination of this type of patient from the clinical studies has greatly reduced this type of reaction.
Some patients have noted a tranquilizing or sedative effect. In most cases this has not been severe enough to warrant concern.
Sedation may occur more in elderly patients with cerebral arteriosclerosis. In the younger individual it occurs more often before meals. It may occur after the first application and, if it is observed, the patient should be cautioned about driving or pursuits that may harm himself or others. Some patients have noted an apparent potentiation of sedatives like barbiturates or alcohol. These findings have not been observed in the laboratory.
Some patients have a garlic or oyster odor on their breath after topical administration of DMSO. There have been a few cases of mild nausea. All of these effects have disappeared when the drug was discontinued.
Blood chemistries have been followed on a large number of patients, and these have not shown significant changes.
Earlier studies included oral administration of the drug. This route of administration is not being investigated at the present time. (Oral and parenteral studies may be initiated at a later date.) These patients received 30 to 60 ml. per day orally for a period of two weeks, and weight loss from 5 to 10 pounds was noted in 50 percent of the patients. This may have been from loss of appetite
Note: aspirin, heparin, and warfarin were in use by 1965 but were not mentioned in this document. It’s hard for me to assess if artificial joint replacements would have been evaluated since the technology had only been on the market for a few years, especially since on one hand patients with replacements would be more likely to enroll in these trials (due to complications from the surgeries) but simultaneously, may have been less attractive clinical trial investigators (since the technology was still moderately new).
In each of the studies I’ve looked at, the authors consistently noticed a lack of side effects, excluding irritation of the skin, a garlic odor, occasional nausea, and vomiting, and once a large enough sample size exists, the 1 in 1-2000 risk of an allergy to DMSO. Additionally, when DMSO is given intravenously, there is often a temporary slowing of the heart rate, and in some cases, either an osmotic hemolysis of weaker (older) blood cells when DMSO is used at higher concentrations (30-40%) is infused intravenously (which often causes blood urine but does not affect kidney function), or significant urination and in some cases a fluid overload or hypernatremia when low concentrations (below 10%) are used. Note: this concentration dependent effect of IV DMSO led to a variety of research to determine the optimal dose that is without either of these issues. When we use intravenous DMSO in practice, we use a fairly low concentration and have not run into the fluid overload or hypernatremia issue (which I believe is due to us using a much lower total dose of DMSO). Likewise, doctors who use higher concentrations of IV DMSO will evaluate a patient’s blood count throughout the treatment to ensure they don’t cause hemolytic anemia.
In the most extensive safety study conducted on DMSO (done in cooperation with the FDA from 1967 to 1968), from a pool of 400 volunteer prisoners, the healthiest volunteers (e.g., no pre-existing conditions) were selected to either be the 33 controls or to be the 78 who received 80% DMSO gel given at 3-30 times the normal dose (done by stripping them and covering their entire body with DMSO) each day for either 14 or 90 days, all of whom were then monitored on a daily basis by a large team of doctors (e.g., many specialists). Alongside regular physical examinations, the subject’s blood work, eyes, EEG, bone marrow, EKG, and cerebral spinal fluid were routinely assessed.
From this large volume of data, the only abnormality detected was an occasional transient blood work change, but except for a transient (likely histamine-induced) increase in eosinophils during the first few weeks (which occurred in 23 [51%] of the 45 DMSO treated subjects) and 8 (31%) of the controls, none of these changes appeared to be related to DMSO.By far the most common side effects were skin irritation or a garlic-like odor. Additionally, the following side effects were reported in the 65 subjects who used DMSO (at an impossibly high dose) for 90 days:

Note: many of the prisoners in the study also self-reported an improvement of existing chronic pain conditions. Additionally, most of the subjects who left the study (which overall had a low dropout rate) did so because they were moved to another prison, they wanted to be paid more for participating, they did not like the odor, or they did not like the skin irritation (although many who experienced those symptoms continued).
One large meta-analysis tried to compute the risks of DMSO. While its results are generally in accordance with what I described (i.e., nausea is a common side effect of IV DMSO), many of the studies I reviewed were not included in it, and instead, while this review had some DMSO only studies, it was predominantly composed of studies where DMSO was used in conjunction with something else (most commonly stem cells, followed by topical diclofenac DMSO was used to bring into the system, followed by Onyx, a polymer that is used repair ruptured arteries under anesthesia and thus represents a much higher risk situation than when IV DMSO is typically used). Because of this, the risks that the review showed of adverse events, while low, were significantly higher than what I observed in the individual DMSO studies I’ve looked at (e.g., this study, this study, this study and this study of IV DMSO all either reported there were “no side effects” or “no significant side effects” from the therapy). Likewise, I believe this mix of DMSO containing agents explains why the sample sizes varied for each symptom that was reported.


Note: the two cases of asystole (cardiac arrest) occurred when DMSO was to patch ruptured blood vessels. To quote the study: “bradycardia was observed in 4 cases, with a brief asystole in 2 of these patients during transarterial and transvenous Onyx delivery at cavernous sinus and orbital levels [which reversed with cessation of the injection and atropine—a drug that reverses parasympathetic activity]. Based on our observation, hemodynamic instability was demonstrated during Onyx injection into the vessels that were in close proximity to the trigeminal nerve or its branches, especially in low-flow/low-volume compartment and may represent a direct effect of dimethyl sulfoxide/Onyx on the trigeminal nerve, resulting in vagal response from trigeminocardiac reflex.”
Additionally, I have also found a few other reports of Onyx (or stem cells combined with DMSO) causing cardiac arrest—but I do not believe these instances are applicable to normal IV DMSO administration, except for a minor slowing of the heart (which likely results from DMSO increasing parasympathetic activity), nothing comparable to these incidences was ever reported with just IV DMSO alone.
Similarly, to quote another review paper which examined the effects of infusing DMSO preserved stem cells:
A retrospective review of the published literature identified several hundred adverse reactions (e.g. nausea, chills, cardiac arrhythmias, neurological symptoms and respiratory arrest) associated with the transplantation of stem cells cryopreserved with dimethyl sulfoxide. The occurrences of these are generally accepted as commonplace, as the majority of reactions are transient, whilst a few patients may require clinical treatment.
Note: this paper also found these reactions were proportional to what was infused, how fast it was infused and how much in total was infused (as did this one), while another review noted these reactions could be mitigated by mixing saline or albumin into the infusion and another trial found the nausea and vomiting could be relieved by sucking orange flavored lollypops. When IV DMSO (without the other additives) is given in practice, nausea is sometimes reported, and likewise, lowering the drip rate of stronger solutions can reduce discomfort, so the insights gained from using IV DMSO with stem cells may be useful for using IV DMSO alone.
FAERS
FAERS is used by the FDA to track adverse reactions to drugs, and like VAERS, only receives a small fraction of the reactions that occurred (estimates range from 1-10%) and typically thousands of reactions and deaths (if not tens of thousands) have been reported to it for many commonly used drugs. Since 1980, 214 reactions to dimethyl sulfioxide (including 21 deaths) were reported.
Of the reactions, 101 came from DMSO. In contrast, 113 came from DMSO with something else, which included eight cases of Onyx triggering the trigeminal cardiac reflex or asystole (with numerous published case reports being attached to the FAERS reports) along with a few cases of stem cell transplants causing significant issues and 3 allergic reactions which may have been linked to DMSO. Of the 101 where DMSO was attributed as the cause, 27 involved another drug which might or might not have been responsible for the reaction, and based on what happened in those 101 reactions, I suspect unlisted drugs played a role in other cases too.
In those where DMSO was the apparent culprit, 10 deaths occurred, but very little information was provided for each case. Of them, 1 also mentioned an anaphylactic reaction, 4 “hemolysis and hematuria,” 1 “ coronary artery occlusion,” 1 “injection site reaction,” 1 “hypernatraemia,” 1 “gangrene; sepsis” and 1 (which was also published at this conference) listed a variety of conditions. The remaining 94 non-fatal cases included 19 skin reactions (including 4 characterized as “dermatitis bullous” and 1 as urticaria), 16 harmless product administration errors (e.g., given during pregnancy, drug ineffective, or an accidental exposure to the product), 12 gastrointestinal issues (e.g., vomiting), 8 eye issues that didn’t appear to be adverse reactions (4 lazy eyes, 3 cataracts and 1 “eye disorder”), 7 anaphylactic reactions, 7 “pain” (e.g., from DMSO being put into the bladder), 7 other cases where the bladder or vagina reacted to DMSO (e.g., pain or irritation), 6 fevers, 6 headaches, 5 cases of weakness or malaise, 4 changes in taste (e.g., loss of taste), 4 shortness of breath, 3 other eye issues, 3 with facial edema, 3 with nausea, 3 that did not appear linkable to DMSO (e.g., an un-evaluable event, a variety of chronic conditions unrelated to DMSO or a suture rupture), 3 with dizziness, 2 with breath odors (and one that also had a change in smell), 2 with seizures, 2 with tachycardia, 1 with hematuria, 1 with TTP, 1 “non-serious” encephalitis, 1 “respiratory disorder,” 1 chest discomfort, 1 pruritus with elevated bilirubin, 1 case of low blood pressure and 1 case of fainting, 1 with confusion, 1 with chills, 1 with flushing, and 1 with muscle pain.
Most of these effects were consistent with what’s been attributed to DMSO, some of them were likely unrelated to DMSO, and overall, given how rare they were, they collectively suggest DMSO has a very low toxicity. Additionally, the pain and discomfort experienced when it is put into the bladder is to be expected as the primary approved condition it’s used for is characterized by immense irritation and pain in the bladder (which is why in more severe cases of that disease DMSO is given orally rather than directly into the bladder).
Note: I did my best to accurately represent the FAERS data (since it is very time consuming to go through), but there may be minor errors (e.g., some of the above numbers above being off by 1 or 2).
Lens Toxicity
By far the most notorious side effect of DMSO was it allegedly changing the refractive index of the eyes (which is what glasses correct) by decreasing the normal relucency of the lens cortex, thereby causing the normal central zone of the lens to act as a biconvex lens.This controversy arose because dogs were observed to develop this myopic change after receiving 5g/kg of DMSO (roughly fifty times the human dose) for 9 weeks, with the changes typically taking 5-10 weeks to emerge or longer when a lower dose was used. This dose dependent effect was then confirmed to also occur within 90 days in pigs receiving 2.7-4.5g/kg of 90% DMSO twice daily, in rabbits receiving 1g/kg of DMSO a day for 12 weeks (but not when they received 0.1-0.5g/kg) and that rabbits and dogs were more sensitive to it than pigs. These changes progressively worsened over the course of 6 months of DMSO treatment, and gradually reversed once DMSO was discontinued (taking longer to reverse in dogs).
Note: these lens changes did not appear to affect the animals ability to perceive and navigate their environment and when the eyes were dissected, was attributed to the reduction of soluble proteins in the eyes.
When tested in monkeys, 3g/kg of a 40% DMSO solution for 9 days did not lead to any lens changes (or any other pathologic changes) over the next 120 days. Likewise, a dose of 11g/kg for 6 months did not produce any lens changes nor did a dermal dose of 11g/kg or an oral dose of 5g/kg given for 1 year, all of which suggested primates have a significantly greater resistance to this effect of DMSO.Note: beyond not showing lens changes, those studies also showed a complete lack of toxicity from DMSO for the monkeys.
In humans, no lens changes have ever been observed (in contrast many patients, such as those with macular degeneration, report improved eyesight from DMSO). For example, in addition to the prison study (which was designed to definitively answer this question) Stanley Jacob had 32 patients who received an average of 30g of DMSO for 3-19 months receive regular eye exams. The only potential exception to this was a study of 44 patients with scleroderma (a condition which frequently causes changes to the eyes) who received DMSO a 3g/kg for as long as 23 days. Due to the challenges of regularly examining the eyes of these patients (both before and during the study) adequate testing was not performed that could have definitively proven the eye changes they had were a result of scleroderma rather than DMSO (although the eye changes that occurred differed from the refractive changes observed in dogs, pigs and rabbits).
Note: after the 1965 testing ban, many pharmaceutical companies continued to collect case reports on patients using DMSO (Merck collected approximately 17,000 cases, Syntex 7,000 cases, and E. R. Squibb and Sons 3,000). No toxicity was detected by any of these companies, including changes in the eyes when DMSO was given at 11 g/kg dermally and 5 g/kg orally per day for a year. Additionally, in 1971, a committee from the National Academy of Sciences submitted a report to the FDA that stated DMSO had a “relatively low toxicity level,” apart from the unexplained eye effects in certain animals.
Teratogenicity and Genotoxicity
A key aspect of testing a new drug for safety is to assess if it can cause either cancer or birth defects (which the mRNA vaccines were exempted from and we in turn are now all suffering from as the spike protein is highly carcinogenic).
In the case of DMSO, it was determined that in certain animals, directly injecting high concentrations of DMSO into the vicinity of developing embryos could cause birth defects, but these effects were not observed at lower doses, or when DMSO was taken orally and not seen in all animal species. Specifically:
•A 1967 study injected chicken embryos (that were either 72 hours or 96 hours old) with toxic doses of DMSO and found that as the LD50 was approached, malformations would frequently occur in the chicks that survived (e.g., 25.9% of the surviving embryos which had a toxic dose of DMSO at 96 hours then developed defects).
Note: a 2021 study also found that injecting too high a concentration of DMSO could cause birth defects or kill chick embryos (whereas at lower doses no effects were noted).
Since previous experiments with lower doses of DMSO had not been observed to cause birth defects in mammals, mice, rats, and two species of rabbits were then given 50% DMSO (either orally or through abdominal injections into the animals) from the 6th to the 12th day of gestation and then dissected a few days before their scheduled delivery.•In mice, no changes were observed in the rate of abortions, and no birth defects resulted from oral DMSO, but when DMSO was injected into the abdomen, 7% of mice developed birth defects (compared to a typical rate of 0.226%) •In rats, injecting DMSO was found to increase the rate of abortions, and reduced the birthweight of living rats by 15.4% (dosed at 8g/kg) to 28.5% (dosed at 10g/kg), and 1.5% developed birth defects (compared to 0.2% of controls).•In rabbits however, no effects were observed from oral or injected DMSO.
•Another study found intraperitoneal injections of DMSO (at 5.5 g/kg) into pregnant hamsters could cause developmental malformations of their embryos. Likewise, another hamster study found injecting 0.5ml of DMSO intraperitoneally into hamsters on the eighth day of gestation caused varying degrees of exencephaly and an encephaly (brain changes).
In one study [I could not locate], eight cell embryos were soaked in DMSO and re-implanted. All developed normally. Indeed, DMSO is by any measure one of the least embryo-toxic substances in pharmacology.
It is routinely used as a solvent when scientists are studying the mutagenic effects of other drugs. DMSO’s nonmutagenic effects have been confirmed by a scientist named Bruce Ames, whose test is the standard by which the FDA itself measures mutagenicity.
DMSO has also been successfully used to treat infertility without issue. For example, in this study, 47 women who were sterile (e.g., due to a tubal obstruction) received DMSO, with 27 (57.4%) then becoming pregnant. Of the 27, 12 had healthy full term babies, 7 were still continuing the pregnancy at the time the study was published, 4 elected to have voluntary abortions, while 3 had spontaneous abortions (and no other issues were reported). Given that these were high risk pregnancies, the fact only 1/9th of them ended up in spontaneous abortions (lower than the expected rate) and that no other issues were reported, this argues for DMSO’s safety in pregnancy.
All of this in turn suggests that DMSO as typically used is not teratogenic (e.g., it’s never injected into the belly), but since it was never formally tested the DMSO community always advised avoiding it during pregnancy since they could not guarantee the risk was 0. That said, within the scientific literature, no cases of any toxicity to the offspring of animals topic skin applications of DMSO have ever been reported.Note: many commonly used pharmaceuticals can cause birth defects. For example, as I showed here, SSRI antidepressants (which are often pushed on mothers during pregnancy) double the risk of premature birth, increase the risk of a septal defect (which requires surgery to repair) from 0.5% to 0.9% (or to 2.1% if multiple SSRIs are taken), and increases the risk of persistent pulmonary hypertension (which occurs in 1-2 out of 1000 births) by 2.5 to 6.1 times (see this study, this study and this study). In contrast, I do not know of a single case where DMSO was shown to have caused a human birth defect.
Finally, as discussed in the first part of this series, rather than damage DNA, DMSO tends to protect it from damage (e.g., see this study, this study, and this study) additionally, as I will discuss later in this series, DMSO has also been shown to treat cancer by both causing cancerous cells to become normal cells or slowing their growth, and to significantly increase the ability of a variety of agents to kill cancerous cells (while simultaneously protecting normal cells from damage). Presently, I have not come across any studies showing DMSO causes DNA damage in normal cells.
Note: DMSO also has repeatedly been shown to have no cancer causing activity.
Additionally, many of DMSO’s remarkable effects come from its ability to stabilize proteins (discussed further in the first part of this series) and dissolve abnormal ones (e.g., amyloids), which in turn likely accounts for why it can cure a variety of incurable illnesses (e.g., genetic ones). In turn, a variety of studies with newer technology have been conducted which show it subtly alters the function and configuration of proteins within cells (e.g., see this study, and this study). This in turn, has led the authors of this newer research to state the longstanding assumption that DMSO is “inert” may not be correct, and to assume there is the potential some of the changes DMSO creates may be problematic or destabilize proteins—an assumption which I believe arose from the fact those authors were unaware of the literature showing that DMSO instead stabilizes proteins.
Other Potential Issues
I would like to conclude this section by disclosing all the other potential issues with DMSO I have come across over the years:
•Three of DMSO’s characteristic effects (a rapid improvement of a patient’s symptoms, the garlic like odor, and the frequent irritation of the skin) make it immensely challenging to conduct blinded trials where patients are unsure if they did or did not receive DMSO. This ultimately was what created the biggest problem for DMSO.
•Sensitive patients or those with liver congestion can experience a Herxheimer reaction to DMSO (e.g., fatigue or headaches) which at most lasts for 12-24 hours due to DMSO accelerating the detoxification process (e.g., one sensitive reader shared that 8-12 hours after using DMSO they would get a moderate headache)—a process which I suspect is partially mediated through a release of histamine. Within the DMSO community, it’s thought that these reactions can be mitigated by using a lower DMSO dose or aiding the detoxification process (e.g., with rest, fasting and drinking reverse osmosis water) and that it will often decrease in time as the body has detoxified itself.
Note: individuals who react to other sulfur compounds typically do not react to DMSO or MSM.
•Clinically, umbilical cord blood stem cells or exosomes that are frozen without DMSO perform tend to perform better than ones that were frozen with DMSO (although DMSO preserved ones still work).
•While DMSO is typically non-toxic and most surfaces of the body can tolerate appropriate concentrations of it (e.g., the eyes and the ears), a study found that rabbits who inhaled 25-50 ml/hr of DMSO for an hour each day for 8 weeks developed pathologic changes in the liver and lungs. While this was a high dose, nebulizing DMSO has nonetheless been advised against and very little information exists on if it can be done safely.Note: this is somewhat analogous to how ozone can be injurious to the lungs, so while many different routes of administration exist for medical ozone therapy, inhalation is never done.
•DMSO is flammable and can cause explosive decomposition reactions when mixed with certain chemicals. This is unlikely to come up in home use (especially if you do not expose it to an open flame) but has caused numerous industrial and laboratory accidents.
•When giving DMSO intravenously (especially at higher concentrations) it can partially dissolve plastics that are not DMSO resistant. For this reason, it is important the correct materials come into the contact with it.
•One forgotten cancer cure the AMA wiped off the earth were the Koch Catalysts. I was advised by the people who gave them to me, that low doses of solvents could inactivate them (e.g., a patient on them should never pump gasoline), and that DMSO could also do inactivate them. Given how difficult the catalysts were to obtain and how limited my supply was, I hence always made sure anyone who used them did not also use DMSO.
•DMSO can be manufactured from either wood pulp or a petroleum source. I have seen some evidence suggesting people have a different therapeutic response depending on which source they use, but not enough to be certain one is preferable to the other. For this reason, if any of you have the opportunity to try more than one of the brands I recommended and you notice different effects from the same concentrations, please share them with me.
•While I have not come across any major issues arising in people taking non-medical grade DMSO (e.g., DMSO from the hardware store) there are a lot of theoretical reasons why this is a bad idea to do. For this reason, I strongly recommend getting one of the widely available high-purity brands people have used for years without issue.
Conclusion
One of the particularly unfortunate aspects of human society is that humans typically cannot take a broad view which takes into consideration all the pertinent data and instead will hyper focus on what they have been primed to care about. This for example is how the medical industry was able to not only sell but mandate the COVID vaccines to the public (which did not work and were far more dangerous than COVID-19) as all the marketing around the vaccines:•Greatly exaggerated the risk of COVID-19.•Disclosed the benefits of the COVID vaccines as relative benefits (which obscured the fact a serious complication of COVID-19 was so rare it was highly unlikely you could ever benefit from a vaccine preventing it).•Kept moving the goal posts on the COVID-19 vaccines each time they failed to deliver what had been promised.
•Continually covered up the immense damage the COVID vaccines did to society.
As a result, while many believers in the orthodoxy eventually were red-pilled, we still have many scientific “experts” who have now gotten 6 or more COVID-19 vaccines.
That same issue sadly exists with many other drugs. For instance, beyond DMSO being far more effective than NSAIDS (which are routinely used for many of the musculoskeletal and chronic pain conditions DMSO treats), it is so much safer than them the risks can’t even be compared (e.g., while DMSO has not been linked to a single death, NSAIDS kill tens of thousands of Americans each year and seriously injure far more). Yet despite this, NSAIDs are given a pass, and many sincerely believe DMSO is a deadly poison (not unlike what happened with ivermectin—something the FDA also successfully rebranded as snake oil that only worked in horses but not humans).
Note: one of the things I consider to be particularly tragic with DMSO is how much cruel and completely unnecessary animal testing was done to refute the FDA’s unwavering belief DMSO was dangerous. For example to quote Stanley Jacob: “DMSO has been responsible for the unnecessary death of more laboratory animals than any other drug in the history of medicine.” Yet despite all those deaths (which resulted from massive doses orders of magnitude greater than what any human would ever take), since they demonstrated DMSO’s incredible safety and thus didn’t show what the FDA wanted, they were ignored—a situation not that different from how both the FDA and CDC have adamantly refused to consider the tsunami of evidence the COVID vaccines are incredibly dangerous and meet every possible criteria for an emergency withdrawal from the market.
In the second half of this article and the context this toxicology data provides, I will chronicle the entire history of exactly what the FDA did to DMSO (before moving on to its incredible utility for a variety of other challenging conditions). In the meantime, I request that if you have any stories from your experiences with DMSO, please share them in the comments here.
Lastly, for those wishing for additional resources on DMSO, in addition to reader testimonials which can be found within this article and this article and numerous comments within this Twitter thread and this Twitter thread, the previous two parts of this series can be read here:
Click below to share this article!
To learn how other readers have benefitted from this publication and the community it has created, their feedback can be viewed here. Additionally, an index of all the articles published in the Forgotten Side of Medicine can be viewed here.To learn how other readers have benefitted from this publication and the community it has created, their feedback can be viewed here. Additionally, an index of all the articles published in the Forgotten Side of Medicine can be viewed here.




